Products
Quality Management
Quality Assurance System
We have established our quality policy in accordance with the "Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices" (hereinafter referred to as the "PMD Act") and related laws and regulations, as well as ISO 13485:2016 (International standard of quality management systems for medical devices).
In the event of a problem with products, we will identify the cause and implement corrective measures in accordance with the PMD Act and related laws and regulations, as well as our own quality management system. We also disclose information on our website about problems where the use of products could cause serious health problems or death.
In May 2023, we also obtained MDSAP (Medical Device Single Audit Program) certification since we are looking at expanding our in-house products overseas in the future. MDSAP is a single audit program that certifies compliance with the quality management system regulations of the member countries (U.S.A., Australia, Brazil, Canada, and Japan).
Product Quality Management
Most of the medical devices that we handle are classified as "highly controlled medical devices" that are used on the human body, and thus require stricter control under the law. For this reason, we have established procedures for product safety in accordance with the PMD Act and related laws and regulations, and ISO 13485:2016, and have obtained ISO certification at our head office and each factory.
In the manufacturing process, we strictly enforce the prohibition of bringing unnecessary items for the work into the workroom, and the prohibition of work that is not described in the manufacturing process control procedures. In addition to conducting multiple inspections at the work site to ensure product quality, our Toda Factory and Oyama Factory have installed work site monitoring equipment in each factory to ensure a higher level of quality control. If a product is found to be defective and is expected to have an impact on quality, efficacy, or safety, it is reported to the government authorities as a recall/refurbishment.
In order to sell medical devices in Japan, we confirm that the quality, efficacy, and safety of them are kept appropriate and sufficient, and sell them only after obtaining pharmaceutical approval from the administrative authorities.
Handling Complaints about Products
As for opinions including complaints about our products from medical professionals who use our products, we obtain them through our sales representatives and website. This information is managed by the Quality Assurance Department through an internal system and shared so that the person in charge can check the contents as necessary.
As for products, all information reported by sales representatives is fed back to the factory for investigation. On the factory side, we have a system in place to check for problem by tracing manufacturing and inspection records. With regard to products, we request the manufacturer to investigate and confirm, if necessary.
The Marketing Department obtains information on consultations and inquiries and feeds it back to the relevant departments.
Through the PDCA cycle described above, we make use of feedback from relevant stakeholders for quality improvement.
Considerations in the Provision of Information and Labeling of Products
We comply with PMD Act and related laws and regulations in the preparation of attachments and catalogs for the products we handle, as well as in the provision of information and labeling.
Research and Development
Our R&D department has about 90 employees. Development based on market research by the Marketing Department and development based on the results of elemental technology research by the Development Department is carried out, and R&D is managed in stages according to the "Design and Development Procedure."
In order to catch up with the latest trends in medical devices and apply them to R&D, we send our staff to international conferences, exchange information with medical professionals who visit our factories, and share information with the marketing department.
The R&D Division has established policies such as "R&D with Originality," "R&D with an Awareness of Clinical and Manufacturing Aspects," and "Timely Provision of Products that Meet the Needs." We are working on the development of new products and the improvement of existing products, as well as R&D themes aimed at improving the QOL of patients, a universal social issue.
Open Innovation Initiatives
To keep contributing to the society through the creation of better products, we provide doctors and academic societies with information on products that our technologies are used. We have also maintained a policy of actively cooperating with external parties to utilize technologies that we do not have in our company for product development.
As one of our open innovation initiatives, we have developed an artificial tissue gel for ablation evaluation in collaboration with an external R&D organization. (Results were presented at the 2nd Symposium of “the Implantable/Wearable Medical Device Co-Creation Consortium.”) We have also conducted joint research with a domestic extruder manufacturer to develop an original extrusion device for catheters. In addition, we have jointly developed a highly original fiber with a fiber manufacturer as a base material for artificial blood vessels, in which the outer and inner surface have very different structures. Artificial blood vessels using this fiber have been highly evaluated for their excellent hemostatic properties.
Policy on Animal Testing
In the development of medical devices, there are many situations where animal testing is essential. When the use of experimental animals is necessary for unavoidable reasons, we outsource the work. The selection of contractors is based on "The Three Rs" ("Replacement" (use of alternative techniques,), "Reduction" (reduction of the number of animals used to a minimum), and "Refinement" (making sure animals suffer as little as possible)). In addition, the tests will be conducted at facilities that comply with "The Act on the Welfare and Management of Animals", "Basic Guidelines for the Conduct of Animal Experiments in Implementing Agencies Under the Jurisdiction of the Ministry of Health, Labour and Welfare", and "Standards relating to the Care and Keeping and Reducing Pain of Laboratory Animals" noticed by the Ministry of the Environment.
Universal Design Initiatives
In our R&D, we aim to design products that are easy for everyone to use. Whenever we make improvements that affect operability, we always conduct evaluations of operability using touch samples and simulated blood vessels by doctors and technicians who are users and reflect the results in product design. We also conduct evaluations based on IEC62366:2007 (International standard of application of usability engineering to medical devices) when necessary.
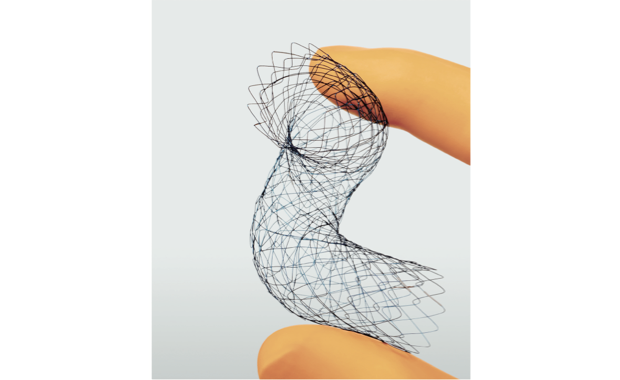
As a result of these efforts, the internal atrial cardioversion catheter "BeeAT", the open stent graft "FROZENIX" "FROZENIX 4 Branched", and the gastro-duodenal stent "JENTLLY NEO" received "Good Design Award" for their unique designs that reduce the physical burden on patients.
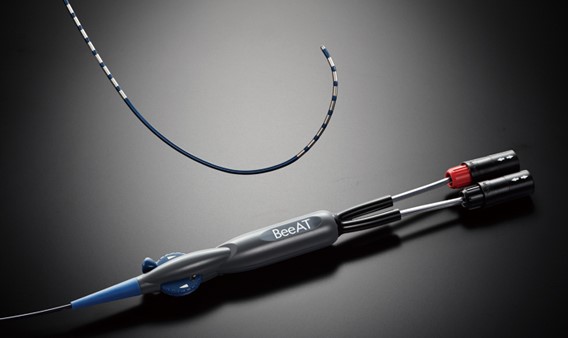
Received the Award in 2018
Internal Atrial Cardioversion Catheter
"BeeAT"

Received the Award in 2019
Open Stent Graft
"FROZENIX"
Received the Award in 2022
Gastro-Duodenal Stent
"JENTLLY NEO"

Received the Award in 2023
Open Stent Graft
"FROZENIX 4 Branched"
Development of Easy-to-Use AEDs (Automated External Defibrillators)
Because AEDs are used in emergencies, they must be designed to be easy for anyone to use. For the AED "Cardiac Rescue RQ-6000" sold by us, we have made a request to the manufacturer regarding the specifications. The illustrations on the unit, the flashing lights, and the instructions for use that come with the unit have been also designed for the easy usability of hearing-impaired people.
The product has been approved by a general incorporated association, All Japan Association of Hard of Hearing and Late-Deafened People, to display the "Ear Symbol" as it was designed with the hearing impaired in mind.

AED (Automated External Defibrillators)
"Cardiac Rescue RQ-6000"

Ear Symbol
Policy on Remanufacturing of Single-Use Medical Devices
A single-use medical device (SUD) is a medical device designed and manufactured with the intention of being disposed after a single use. However, as a part of measures to curb medical costs, there is a movement, mainly in the U.S. and Germany, to collect used SUDs, have them properly processed by specialized companies, confirm that they have the necessary performance, and allow them to be used again. In Japan, a system for remanufacturing of single-use medical devices (R-SUD) was established in July 2017 from the perspectives of "ensuring medical safety," "effective utilization of medical resources," and "providing sustainable medical care."
We participate in the Japan R-SUD Association (JRSA) as a special member and strives to keep abreast of the latest trends.

